The Basics of Diesel-Engine Coolant
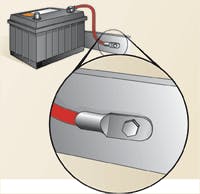
Elizabeth Nelson, coolant program manager at Polaris Laboratories, a fluid-analysis company in Indianapolis, Ind., tells a story that would strike fear into the heart of any fleet manager. A class-8 on-highway truck seemed in fine condition when it left the West Coast, but at the end of its 6,000-mile cross-country run, a cylinder sleeve failed and catastrophic engine failure resulted. According to Nelson, the cause of the disaster was an electrical short in the truck's starter.
"An electrical short in a vehicle takes the path of least resistance to ground," says Nelson, "and often that's through the cooling system. In this instance, the electrical current passing through the coolant so quickly depleted the nitrite additive in the antifreeze, that the sleeves no longer had protection against cavitation. When coolant analysis shows a rapid depletion of nitrite, coupled with an increase in nitrates, it's always a red flag for an electrical short."
A combustion-gas leak, on the other hand, says Nelson, causes a sharp drop in pH (usually below 7) and an increase in sulfates. Air leaking into the engine cooling system, however, typically results in a lowered pH, she says, (but usually not below 7.5) and a drop in nitrite level (but not as rapid as with an electrical problem).
Although regular engine coolant analysis is a good way to keep tabs on the health of a vehicle's coolant system, coolant analysis is not nearly as popular as oil analysis. For many equipment owners, truth be told, what goes on in their machine's cooling system is somewhat of a mystery.
"Ask most fleet managers about their lube-maintenance programs, and they'll talk in detail," says Craig Gullett, brand marketing manager for Old World Industries, a major antifreeze manufacturer. "Ask about their coolant-maintenance programs, however, and more often than not, answers become rather uncertain."
A frequent weak link in engine coolant maintenance, and the probable cause of many coolant-related engine problems, says Carmen Ulabarro, coolants market development specialist for ChevronTexaco, is the lack of understanding about what coolant is being used in the vehicle — and how to maintain that specific formulation.
What's in the radiator?
Virtually all heavy-duty antifreeze is roughly 95 percent ethylene glycol and 5 percent water and additives. The stuff that isn't made from ethylene glycol (only about 1 percent of all antifreeze sold) is made from propylene glycol, which is less toxic, but also more expensive. "Coolant" is created when glycol is mixed with various ratios of water. Typical ratios range from 30 to 60 percent glycol.
Heavy-duty antifreeze formulations differ from one another by virtue of the additive package blended into the ethylene glycol. Additive packages, of course, all have the same task, namely to fight rust, scale and corrosion — and in diesel engines, to protect wet cylinder sleeves from cavitation. But the additive packages among various antifreeze formulations have fundamentally different chemical fingerprints.
Until maybe 15 years ago, heavy-duty engines typically were filled with "conventional" antifreeze, identified by the American Society for Testing and Materials (ASTM) standard D-4985. This antifreeze, however, which is still in prevalent use today, can't be used in diesel engines without first treating it with a "supplemental coolant additive" (SCA) that contains nitrite for protecting wet sleeves. The required initial treatment is an approximate 3-percent concentration of SCA (one pint per four gallons of cooling-system capacity).
Today, the preferred conventional antifreeze for diesel engines is "fully formulated," identified as ASTM D-6210 or RP-329 by the Technology & Maintenance Council (TMC). This antifreeze is sold with an SCA package already blended in, typically including nitrate to protect iron and steel, tolyltriazole to protect copper and brass, borate or phosphate to buffer acids (formed as glycol breaks down), silicate to protect aluminum and nitrite (sometimes accompanied by molybdate) to form a cavitation-resistant barrier on sleeves.
These additives are depleted as the coolant works and ages, however, and must be replenished periodically with an SCA package. Especially critical is the renewal of an adequate nitrite level. But you must be careful here, because too much nitrite may cause solder corrosion, and excess accumulation of other additives causes "total dissolved solids" (TDS) to increase, possibly jeopardizing cooling efficiency and resulting in passage-clogging dropout. Cautious maintenance guidelines may suggest replacing fully formulated conventional coolant at two-year intervals to avoid TDS problems.
To simplify maintenance, the antifreeze industry developed "extended-life coolants" (ELC), which are formulations typically advertised with a service life of 600,000 miles or 12,000 hours. These formulations, originally at least, replaced the additive package used in fully formulated conventional antifreeze with "organic-acid inhibitors," designed to protect metal parts by forming a thin protective skin against destructive forces in the coolant.
These "organic-acid-technology" (or OAT) antifreezes use the base or neutralized version of organic (carbon-containing) acids, typically (but not always) the carboxylate acids of 2-ethyl hexanoic acid (2-EH) and/or sebacic acid. Most heavy-duty carboxylate formulations, however, also contain some of the additives used in fully formulated conventional antifreeze, namely nitrite and molybdate, and sometimes silicate. OAT formulations that include nitrite sometimes are called nitrited-organic-acid-technology antifreeze, or simply a NOAT.
According to some antifreeze experts, anytime you add inorganic inhibitors (like nitrite) to an organic-acid-based formulation, you have created a hybrid, or a Hybrid OAT, or a HOAT. Others say, though, that a hybrid is technically a product characterized by the use of non-carboxylate acids, such as benzoate, from benzoic acid, another organic acid.
Engine coolant maintenance
To ChevronTexaco's Ulabarro's point, the start of good coolant maintenance begins with knowing which antifreeze formulation is in your machine's radiator.
Most NOAT formulations, for example, require the addition of an "extender" at 300,000 miles or 6,000 hours to replenish nitrite, which is used up at a far slower rate in an extended-life coolant than in a fully formulated conventional. Important to note here, perhaps, is that European engine manufacturers are evaluating — maybe even leaning toward — the use of carboxylate-based extended-life coolants without nitrite.
Don't buy into the philosophy, however, that extended-life coolant needs no regular maintenance. The experts recommend inspecting it at the vehicle's regular maintenance intervals to make sure it's clear (no rust), that the color is right (not mixed with another antifreeze type) and that it has sufficient freeze/boil protection, best determined by using a refractometer.
Maintenance guidelines for cooling systems with fully formulated conventional antifreeze typically include periodic testing of SCA levels and appropriate adjustment, as well as periodic draining, flushing and refilling the system to avoid, as already noted, an excess of dissolved solids.
You can test the additive concentration of fully formulated conventional coolant by supplying samples to a fluids-analysis laboratory. Or, you can do the testing yourself by using paper test strips, which are chemically sensitive and change color to indicate freeze/boil point (glycol content), nitrite (or nitrite/molybdate) levels and, in some instances, pH.
When the addition of an SCA is indicated, keep in mind that two major types of SCA are available, one with a nitrite/borate formulation, the other with a nitrite/molybdate/phosphate formulation. It's probably best not to mix them, and it's best to use a test strip designed for the specific formulation. Those in the know say to be careful about buying bargain-priced SCA formulations, which may be inferior. Look for a stated compliance with an ASTM standard on the package, likely D-5752, to ensure that you're buying a quality product.
Some users of fully formulated conventional antifreeze, however, employ a coolant filter charged with an SCA package. This filter/additive assembly is designed to release metered amounts of additives over time and, thus, to maintain optimum levels. As long as testing indicates proper additive levels, and provided that top-up is done with a 50/50 mix of the correct antifreeze and deionized water, the assumption is that fully formulated conventional coolant can last far longer than the often-prescribed interval of two years.
On the other hand, says ChevronTexaco's Ulabarro, some users of fully formulated conventional antifreeze drain and replace coolant every year, but do not test or add SCA packages between those service intervals, thinking that nothing will go wrong in that short time. But, depending on the specific formulation of the antifreeze and on top-up practices, says Ulabarro, critical additives could be depleted in as little as 1,000 hours, potentially leaving the engine virtually unprotected for a long time.
In the everyday world, of course, fully formulated conventional and organic-acid antifreezes are sometimes inadvertently mixed in cooling systems. The primary concern about a mixed system is that the distinctively different additive packages in the two formulations will be diluted to the point that neither has the power to afford adequate protection. You're best off, say the experts, to pick an antifreeze type, take all practical safeguards to avoid mixing it with other types, and conscientiously follow the maintenance strategy recommended for the chosen antifreeze.
How to do coolant analysis
Given the changing chemistry of coolants and the increased demand placed on cooling systems by today's engines, Bryan Debshaw, CEO of Polaris Laboratories, believes that coolant analysis will become an increasingly important tool in preventive-maintenance programs. Coolant analysis not only determines coolant condition, he says, but also identifies other vehicle problems that can show up in the cooling system. Coolant-analysis programs typically are available in various levels (and costs), depending on the number of parameters checked.
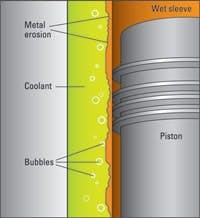
A primary cause of wet-sleeve damage is cavitation, but other causes are prevalent: these sleeves have been attacked by 1) stray electrical current going to ground through the coolant; 2) calcium and magnesium scale that impedes heat transfer and is caused by water with minerals (the sleeve "blues" at 600F); and 3) chloride (in the water), which "decarbonizes" iron until it is like sand, says Elizabeth Nelson of Polaris Laboratories.
Illustration adapted from Baldwin Filter graphic.
ChevronTexaco's Fleet Fix Conversion, Shell's Extended-Life Coolant Conversion, and Old World Industries' Final Charge Converter can be used to make the conversion, but the existing coolant must meet specific parameters before conversion can proceed. (Old World Industries' Final Charge antifreeze is a non-carboxylate organic-acid-based product that contains no nitrite.)
By contrast, Penray, a maker of antifreeze additives, promotes a "Fill-for-Life" strategy, which is aimed at converting nitrited-organic-acid-technology coolants to fully formulated conventional coolant. Penray's conversion strategy employs its Need-Release Filter, which uses corrosion-sensitive barriers for the timed release of SCA charges.
On the other hand, aeration in a cooling system can cause problems that are sometimes blamed on stray currents. Periodically inspect the cooling system for air leaks from loose clamps, bad hoses and bad pressure cap.